Vestimentiferan tubeworms, members of the Siboglinidae family within Polychaeta, represent a remarkable group of deep-sea annelids found in extreme chemosynthetic environments such as hydrothermal vents, cold seeps, and whale falls. Unlike most organisms, these tubeworms lack a digestive system and rely exclusively on a single species of sulfide-oxidizing bacteria for sustenance. The bacteria inhabit a specialized organ known as the trophosome, whose highly vascularized structure resembles that of vertebrate liver lobules. Through this organ, the host tubeworm facilitates gas exchange, delivering essential compounds—hydrogen sulfide, carbon dioxide, and oxygen—that fuel the bacteria’s chemosynthetic production of organic matter.
This unique symbiosis allows tubeworms to thrive in nutrient-scarce environments and form dense, productive communities that dominate deep-sea chemosynthetic ecosystems. For instance, the species Riftia pachyptila is known to achieve remarkable growth, with annual tube length increases of over 85 cm—a rate indicating an exceptionally efficient metabolic process co-evolved by both host and symbiont.
To delve deeper into the molecular intricacies of this partnership, a collaborative research team from the Institute of Oceanology, Chinese Academy of Sciences (IOCAS), Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) and The Hong Kong University of Science and Technology employed single-cell RNA sequencing (scRNA-seq) technology. Their findings, published in Science Advances on July 25th, reveal new insights into the metabolic coordination that sustains this deep-sea organism.
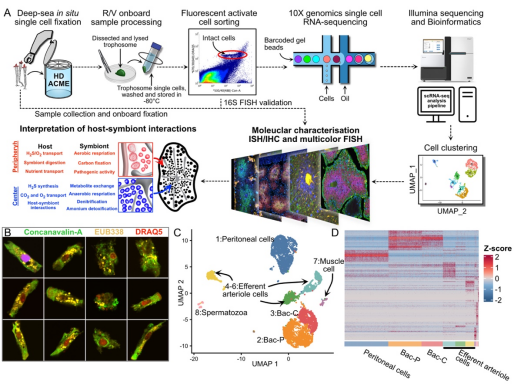
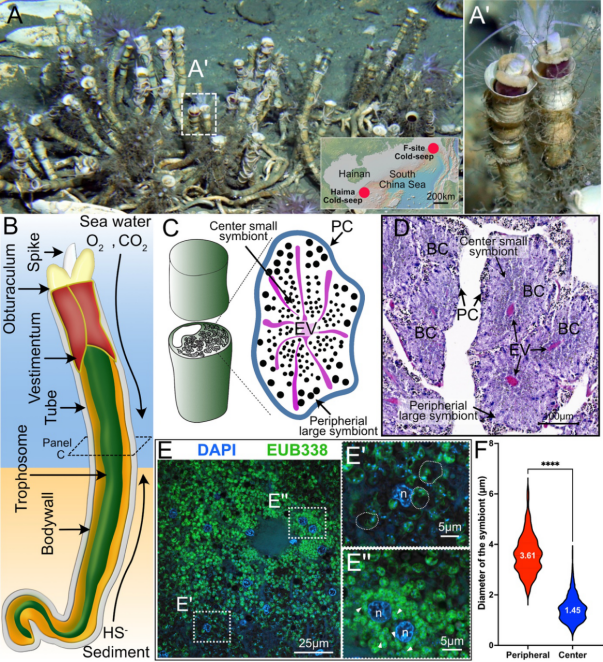
Figure 1. Analysis of the deep-sea Paraescarpia echinospica tubeworm’s trophosome with in situ single-cell workflow
Using a novel in situ single-cell fixation system, the researchers analyzed the trophosome of Paraescarpia echinospica, leveraging the expertise of the "Faxian" remotely operated vehicle (ROV) team. Through scRNA-seq and molecular characterizations, they discovered that the tubeworm precisely controls the distribution of gases and metabolites within the trophosome, creating two distinct metabolic microenvironments: an oxygenated outer layer and a hypoxic core.
In this structured environment, symbiotic bacteria exhibit spatially differentiated metabolic activity. Bacteria in the oxygen-rich periphery perform autotrophic carbon fixation, producing organic material, while those in the hypoxic core engage in anaerobic denitrification, which helps detoxify ammonia waste. This spatial organization within the trophosome reflects a finely tuned metabolic collaboration, maximizing nutrient production and waste management.
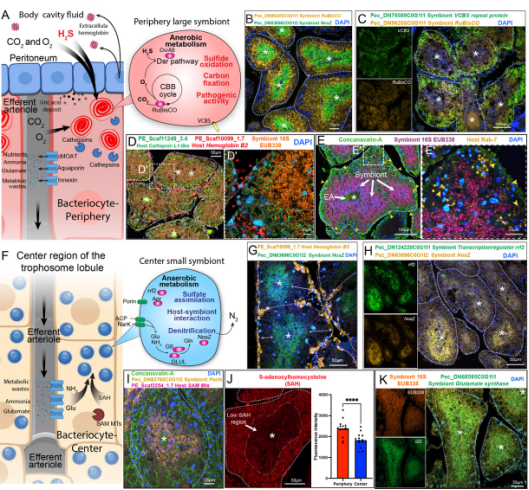
Figure 2. Host-symbiont interactions within the perphery and center “microniches” in the tubeworm’s trophosome loubles.
This study sheds new light on the adaptive strategies of deep-sea organisms and emphasizes the intricate metabolic interdependence between host and symbiont—a relationship that allows these organisms to flourish in some of Earth’s most extreme habitats.
Attachment download:
